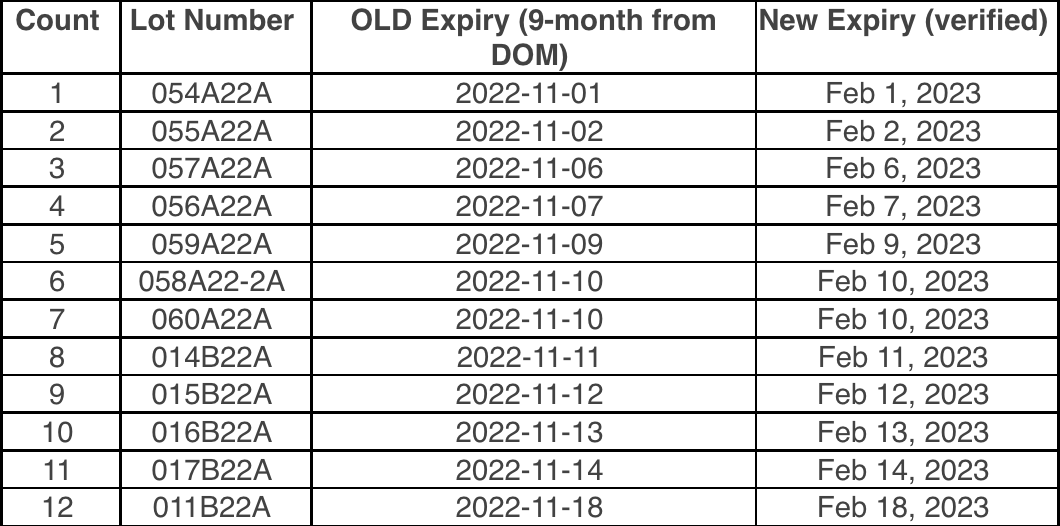
Tubersol Lot Number And Expiration Date 2025 - Tubersol tuberculin purified protein derivative (mantoux) (ppd) (1) for intradermal tuberculin testing is prepared from a large master batch connaught tuberculin (ct68). Tubersol PPD 10Test MDV, 1/Bx, Last updated on may 1, 2025. To aid in the diagnosis of.
Tubersol tuberculin purified protein derivative (mantoux) (ppd) (1) for intradermal tuberculin testing is prepared from a large master batch connaught tuberculin (ct68).
Expiration Dates, Lot Numbers & Batch Codes Enfamil, The generic name of tubersol is tuberculin purified protein derivative. Cdc has additional information regarding the anticipated tuberculin shortage as well as management strategies on their website at.
Shelf Life Extensions on Moderna and Pfizer Products Philadelphia, How to read lot number expiration date. The ifs system is customizable in the way you handle batch labels in your company.

Tubersol Lot Number And Expiration Date 2025. Product now being shipped is from the lot (batch) number shown. The generic name of tubersol is tuberculin purified protein derivative.

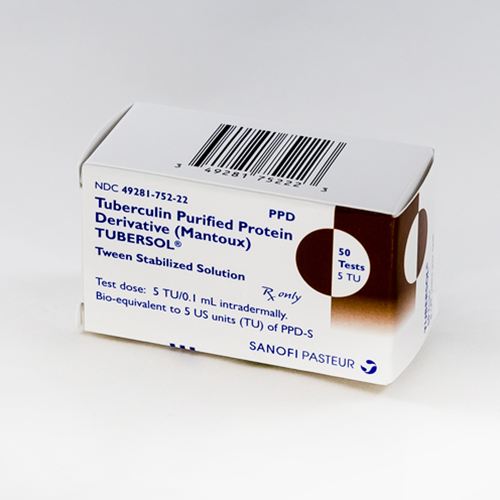
Sanofi Pasteur Tubersol Tuberculin Purified Protein Derivative PPD 5 TU, Protect from light:tuberculin ppd solutions can be adversely affected by exposure to light. Includes common brand names, drug descriptions, warnings, side effects and dosing information.
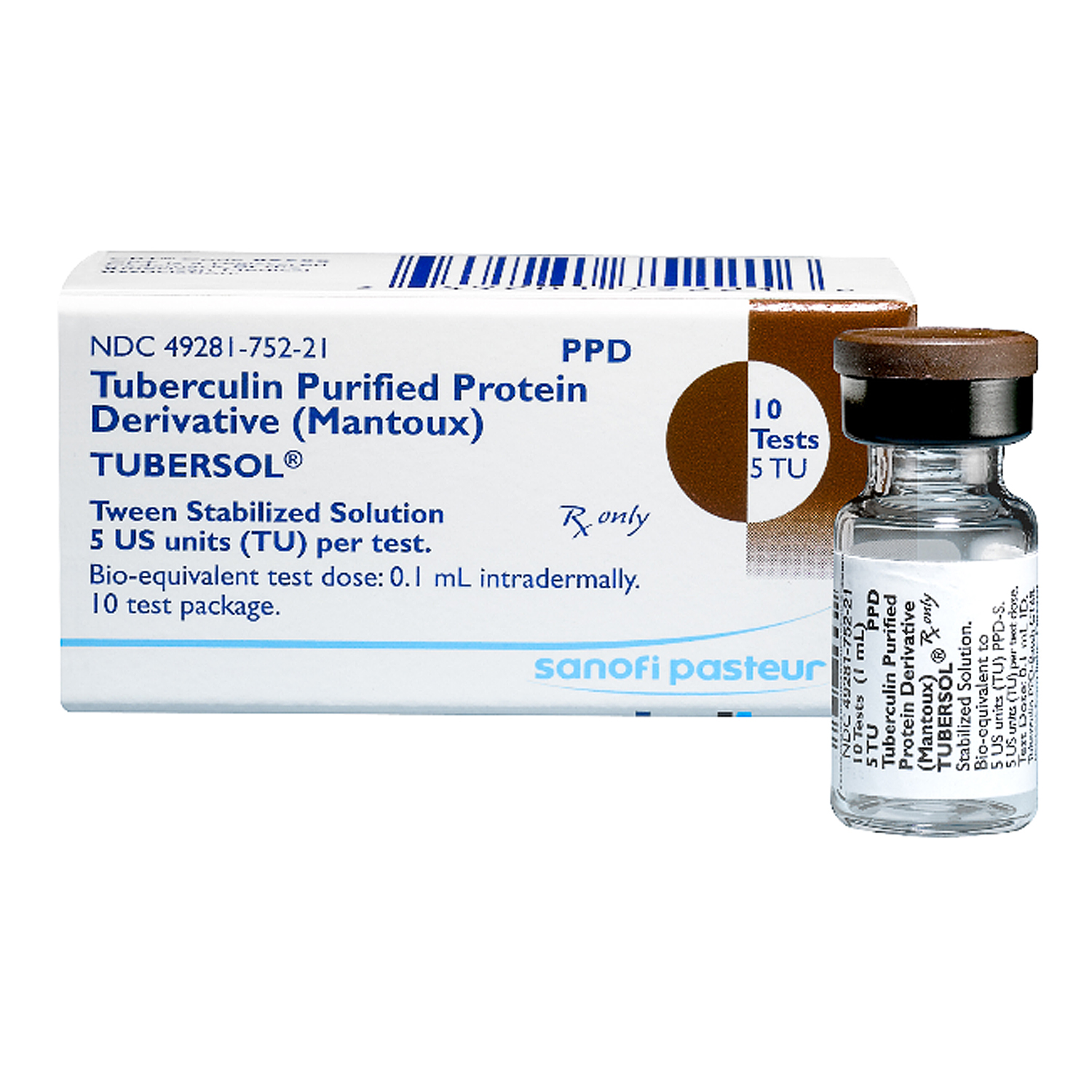
Tubersol TB Test, 5 Tu/0.1mL, 5mL Vial Bound Tree, Includes dose adjustments, warnings and. To aid in the diagnosis of.
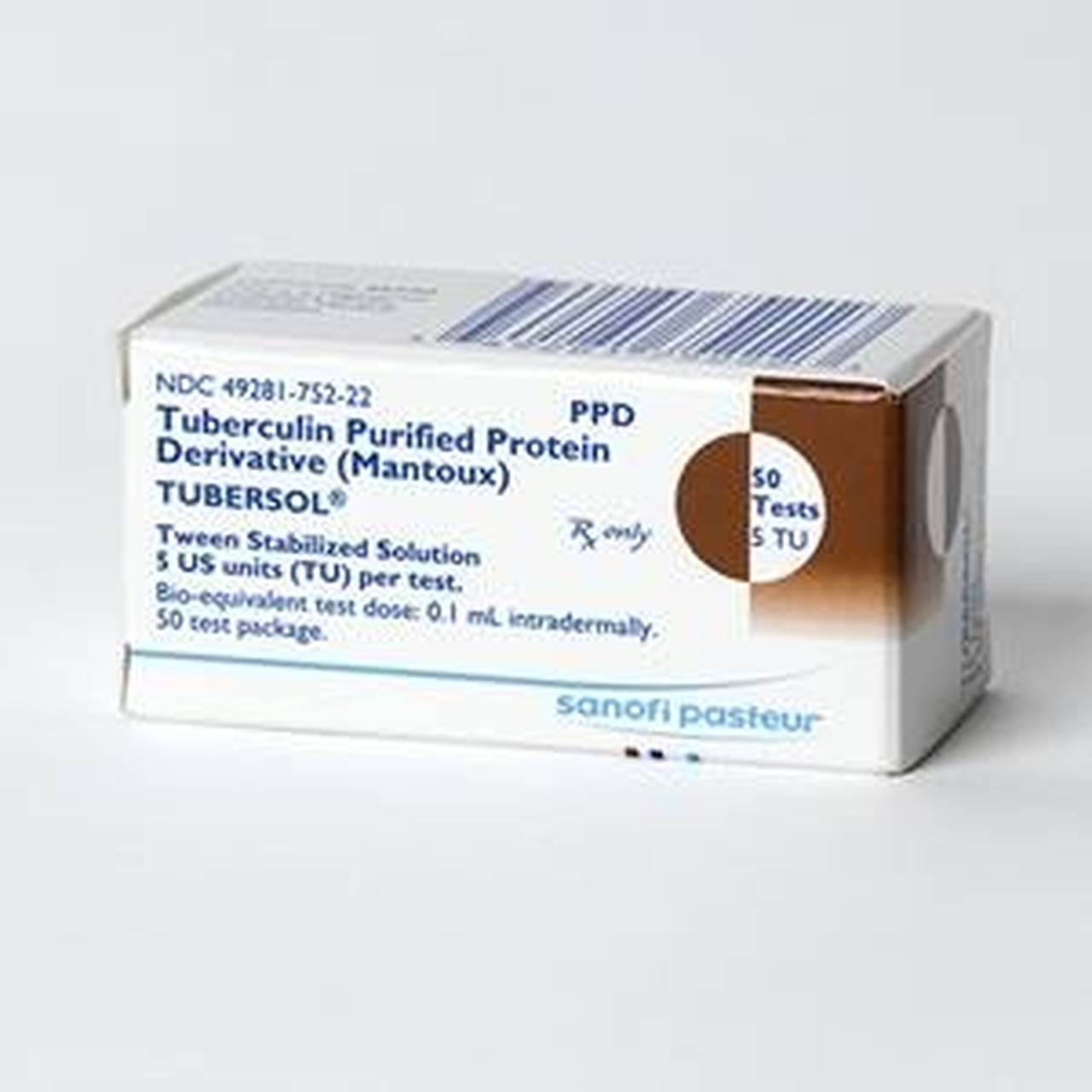
Sanofi Pasteur Tubersol Tuberculin Purified Protein Derivative PPD 5 TU, Protect from light:tuberculin ppd solutions can be adversely affected by exposure to light. A vial of tubersol which has been entered and in use for 30 days should be discarded.
Expiration Dates, Lot Numbers & Batch Codes Enfamil, Tubersol tuberculin purified protein derivative (mantoux) (ppd) (1) for intradermal tuberculin testing is prepared from a large master batch connaught tuberculin (ct68). Product shipped will have the expiry date shown above or a later date.
Aplisol (tuberculin ppd, diluted) is a sterile aqueous solution of a purified protein fraction for intradermal administration as an aid in the diagnosis of tuberculosis.
1.02 PRINCIPLES ASSOCIATED WITH PREPARING OR OBTAINING MEDICATIONS, Store at 2 to 8 degrees celsius (35 to 46. The product's dosage form is injection,.

A vial of tubersol which has been entered and in use for 30 days should be discarded.
Tubersol FDA prescribing information, side effects and uses, Includes dose adjustments, warnings and. Cdc has additional information regarding the anticipated tuberculin shortage as well as management strategies on their website at.
TUBERSOL®, Include name of preparation, manufacturer and lot number, administration technique (i.e., mantoux method), date and dose administered, date of test reading, and extent of. The product, date given, dose, manufacturer, and lot number, as well as the test result in millimeters of induration (including 0 mm, if appropriate).
Detailed drug information for tubersol. The generic name of tubersol is tuberculin purified protein derivative.